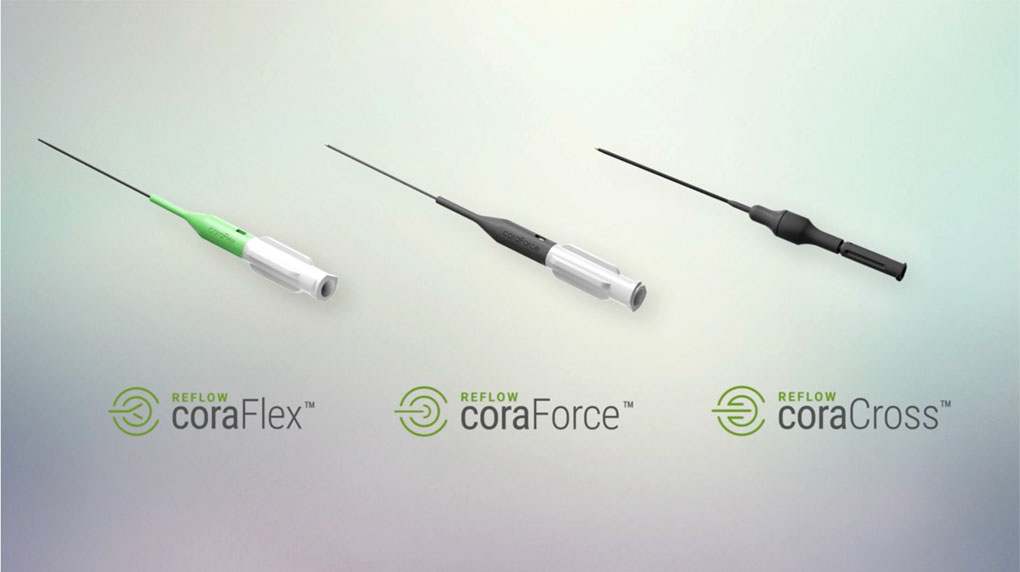
SETTING A NEW
* Data on file
PHYSICIAN IMAGINED. REFLOW ENGINEERED.
A – Multi-layered polymer design with stainless steel inner coils
B – Stainless steel braid with polymer jacket for unmatched torque
C – Uniquely integrated wire coating.
D – No inner liner that could limit torque rotations
Microcatheter features an integrated, flexible, highly radiopaque distal tip:
- First line catheter for antegrade and retrograde approaches
- Optimal navigation in tortuous anatomy including 70–90
degree bends - Lowest overall profile of 2.2F tapering to 2.1F with a 1.4F tip
= less resistance for retrograde with the polymer tip - Crossing and dilating tortuous septals and microchannels

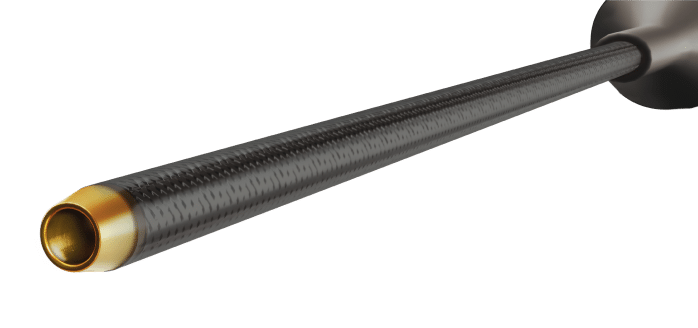
Maximum tip force support for engaging heavily calcified and tortuous lesions:
- Lowest overall profile in the coraCatheter family of 2.2F
tapering to 2.1F with a 1.3F tip - Extra pushability while maintaining a low profile
- Up to 4 times more tip force versus all other polymer
microcatheters*
* Data on file
Patented bevel-extension technology for crossing challenging caps:
-
- Physician-controlled device advancement and activation
- Unique extended beveled tip
- Superior penetration, trackability, and support

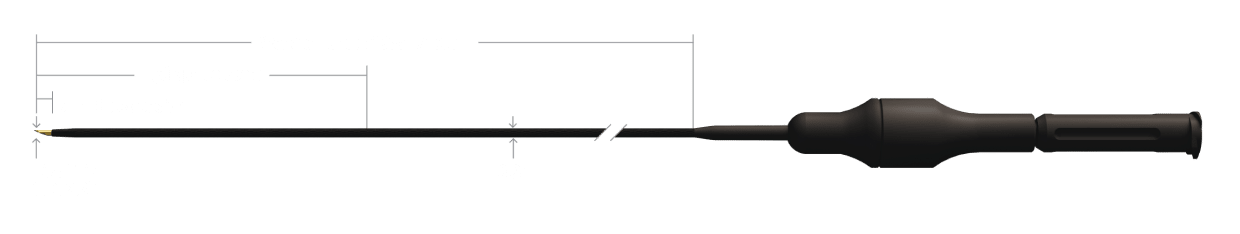



Guide Catheter Compatibility
FLX14135US
MIN 5F (1.7mm)
FLX14150US
MIN 5F (1.7mm)
FRC14135US
MIN 5F (1.7mm)
FRC14150US
MIN 5F (1.7mm)
CRX14135US
MIN 5F (1.7mm)
CRX14150US
MIN 5F (1.7mm)
Guidewire Compatibility
FLX14135US
.014″ (.36mm)
FLX14150US
.014″ (.36mm)
FRC14135US
.014″ (.36mm)
FRC14150US
.014″ (.36mm)
CRX14135US
.014″ (.36mm)
CRX14150US
.014″ (.36mm)
Usable Length*
FLX14135US
135cm
FLX14150US
150cm
FRC14135US
135cm
FRC14150US
150cm
CRX14135US
135cm
CRX14150US
150cm
Sheath Compatibility
FLX14135US
MIN 4F (1.3mm)
FLX14150US
MIN 4F (1.3mm)
FRC14135US
MIN 4F (1.3mm)
FRC14150US
MIN 4F (1.3mm)
CRX14135US
MIN 4F (1.3mm)
CRX14150US
MIN 4F (1.3mm)
Catheter Body Outer Diameter
FLX14135US
.029″ (.74mm/2.2F)
FLX14150US
.029″ (.74mm/2.2F)
FRC14135US
.029″ (.74mm/2.2F)
FRC14150US
.029″ (.74mm/2.2F)
CRX14135US
.042″ (1.07mm/3.2F)
CRX14150US
.042″ (1.07mm/3.2F)
Distal Tip Outer Diameter
FLX14135US
.018″ (.46mm/1.4F)
FLX14150US
.018″ (.46mm/1.4F)
FRC14135US
.017″ (.43mm/1.3F)
FRC14150US
.017″ (.43mm/1.3F)
CRX14135US
.029″ (.74mm/2.2F)
CRX14150US
.029″ (.74mm/2.2F)
*All coraCatheters are hydrophilic coated for 60cm of the catheter body.
Indications, Safety, & Warnings
CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the Indications, Contraindications, Warnings, Precautions, Complications, and Directions for Use.
Indications for Use
coraFlex and coraForce Catheters
Cora Catheters are intended to be used in conjunction with steerable guidewires to access discrete regions of the coronary and peripheral vasculature. They may be used to facilitate placement and exchange of guidewires and other interventional devices, and provide a conduit for delivery of saline solutions or diagnostic contrast.
coraCross Catheter
The coraCross Catheter is intended to be used in conjunction with steerable guidewires to access discrete regions of the coronary and peripheral vasculature. It may be used to facilitate placement and exchange of guidewires and other interventional devices and provide a conduit for delivery of saline solutions or diagnostic contrast.
Contraindications
coraFlex and coraForce Catheters
The Cora Catheter is contraindicated for use in the cerebral vasculature.
coraCross Catheter
The coraCross Catheter is contraindicated for use in the cerebral vasculature.
Warnings
• Single Use only. Do not reuse/resterilize. Reusing the device could result in compromised device performance, cross-infection and other safety related hazards.
• Do not use if device is open or packaging is damaged
• Never advance, withdraw or rotate an intravascular device against resistance until the cause is determined by fluoroscopy.
• Manipulation, advancement, and/or withdrawal past sharp or beveled edges may result in destruction and/or separation of the outer coating, which may lead to clinical adverse events, resulting in coating material remaining in the vasculature or device damage. This may result in adverse events requiring additional intervention.
• Avoid wiping the device with dry gauze as this may damage the device coating. Avoid excessive wiping of the coated device.
• Avoid using alcohol, antiseptic solutions, or other solvents to pre-treat the device because this may cause unpredictable changes in the coating which could affect the device safety and performance.
• Avoid pre-soaking devices, as this may impact the coating performance and has not been tested.
• The safety and effectiveness of the coated device has not been established, or is unknown, in vascular regions other than those specifically indicated
• The performance of the coraCross device has not been assessed in crossing CTOs, especially in the coronary vasculature.
Precautions
• Store in a cool, dry place. Protect from direct sunlight and high temperature.
• Use only appropriately sized ancillary device, as shown in the Specifications below.
• Use the catheter prior to the “Use By” date specified on the package
• The catheter should only be used by physicians qualified to perform percutaneous, vascular interventions.
• Precautions to prevent or reduce clotting should be taken when any catheter is used in the vascular system. Use of systemic heparinization and heparinized saline solution should be considered.
• Exercise care while handling the catheter during procedure to reduce the possibly of accidental damage, kinking or bending.
• Manipulation of the catheter should only occur under fluoroscopy.
Complications
Vascular catheterization and/or vascular intervention may result in complications including but not limited to:
• Vessel dissection, perforation, rupture or total occlusion
• Hematoma
• Hemorrhage
• Arrhythmia, including ventricular fibrillation
• Unstable angina
• False Aneurysm
• Hypo/hypertension
• Thrombosis/Embolism
• Infection
• Acute myocardial infarction
• Renal Dysfunction
• Blood Loss
• Additional surgical or percutaneous intervention
• Radiation Exposure
• Death
©2024 Reflow Medical, Inc. All rights reserved. Reflow Medical, coraFlex, coraForce, coraCross and The Pulse of Medical Ingenuity are registered trademarks or trademarks of Reflow Medical, Inc. Federal law (USA) restricts these devices to sale by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the Indications, Contraindications, Warnings, Precautions, Complications, and Directions for Use.
QUESTIONS?
TALK TO A REFLOW PRO.
For more information, please send us your question or request today.
A Reflow pro will get back to you.