Enrollment Completed in the DEEPER REVEAL Clinical Trial
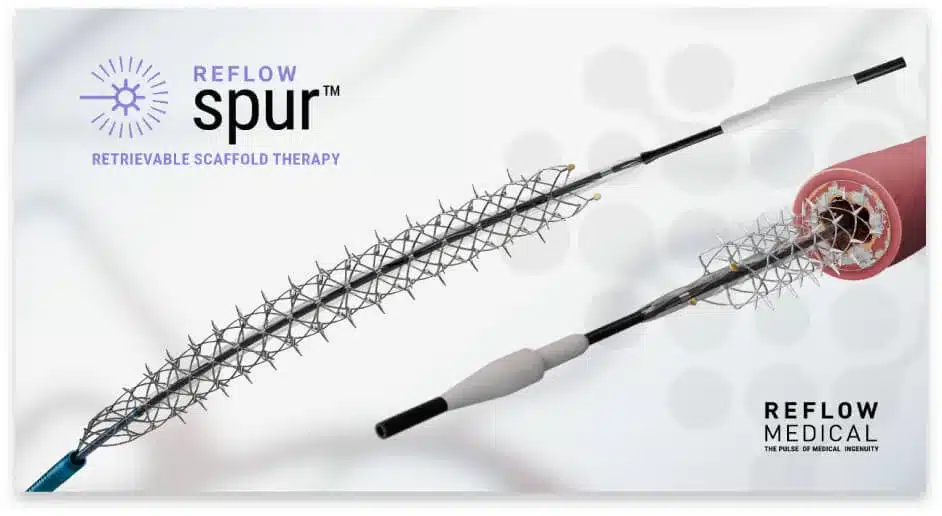 We want to thank everyone who worked to complete enrollment in the DEEPER REVEAL clinical trial (NCT05358353) to evaluate Spur™ Retrievable Scaffold Therapy (RST), which was designated a Breakthrough Device by the FDA. The study enrolled 130 patients in over 35 clinical centers in the U.S.
We want to thank everyone who worked to complete enrollment in the DEEPER REVEAL clinical trial (NCT05358353) to evaluate Spur™ Retrievable Scaffold Therapy (RST), which was designated a Breakthrough Device by the FDA. The study enrolled 130 patients in over 35 clinical centers in the U.S.
Our New European Division: Reflow Medical Europe GmbH
We’re pleased to introduce the members of our newly formed team of professionals in Europe. Knut, Dejan and Harry are working toward the European launch of the revolutionary Spur™ RST and bringing the Reflow Wingman™ and Spex® to physicians in Europe.
Reflow’s Successful Educational Efforts at LINC 2024
Our first-ever educational event at The Leipzig Interventional Course in Leipzig, Germany on May 28 drew a good crowd! “Innovations in CLTI: Exploring Spur Retrievable Scaffold Therapy” was moderated by Jos van den Berg, M.D., Ph.D., with panelists Thomas Zeller, M.D., Marcus Thieme, M.D. and Marianne Brodmann, M.D. Three presentations on Spur Retrievable Scaffold Therapy and a live case were also well attended.
Reflow Team at NCVH 2024

Case Study: Wingman™ Crosses Long SFA CTO
Dr. Rao reports on the successful revascularization of the right SFA and popliteal artery using the Reflow Wingman.
U.S. Limited Market Release: SINC™ Catheters