
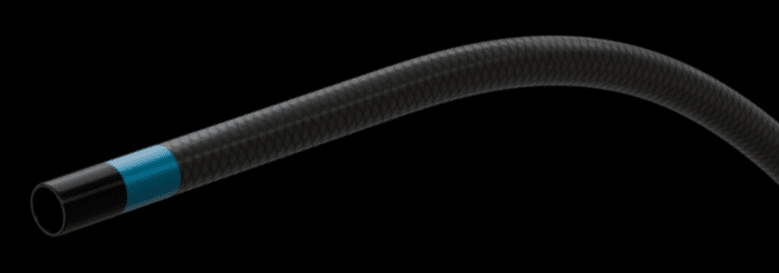
The Lowest Profile Reinforced Support Catheter
- Promotes improved and predictable access to the vasculature
- Features three radiopaque markers that enhance visibility on imaging
- Increases lubricity with a seamless, low-profile hydrophilic coating
- Designed to fill a specific need based on clinical feedback
- Advance engineered for treating complex critical limb ischemia (CLI)
- Optimizes pushability and access through lesions
NEW AND IMPROVED LUER AND STRAIN RELIEF FOR UNMATCHED PERFORMANCE
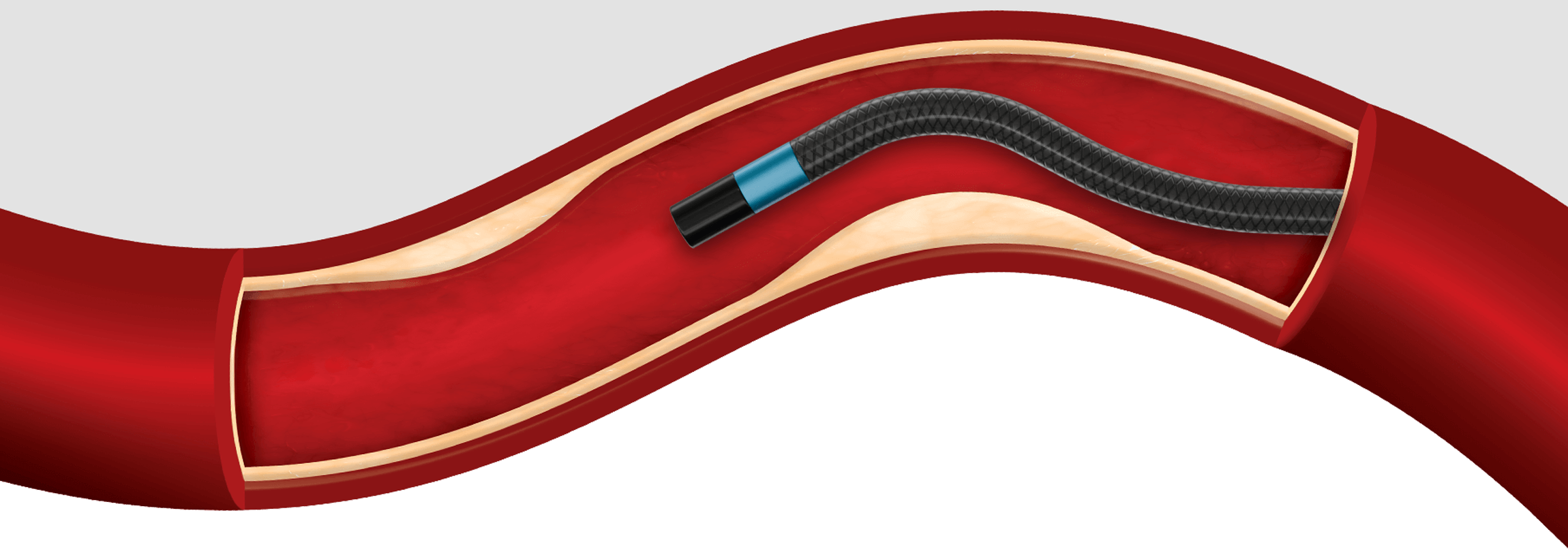
Access and cross difficult lesions with the lowest profile reinforced catheter.
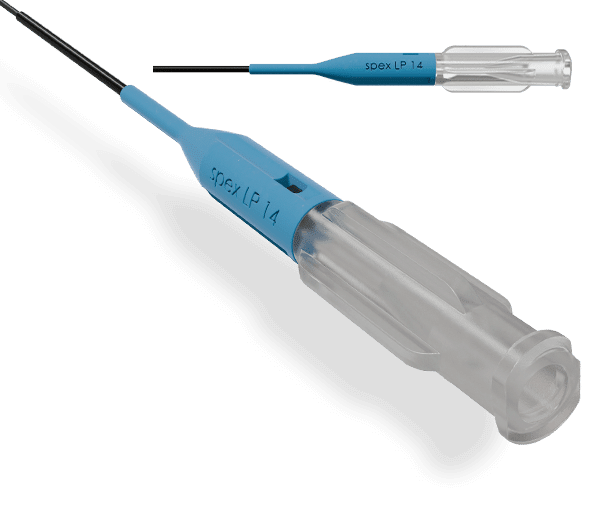
BRAID-REINFORCED MICROCATHETER BODY
Provides added column strength and pushability to cross difficult lesions.
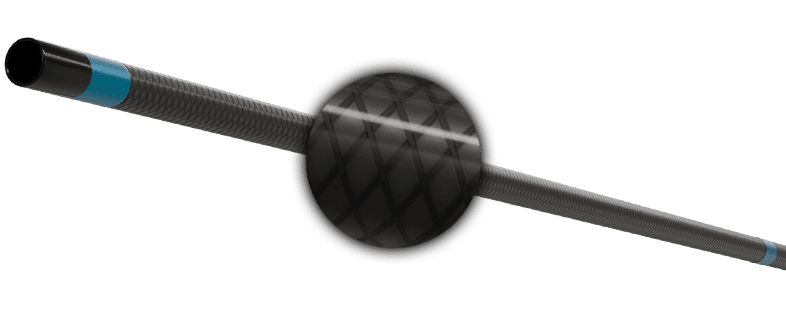
CUSTOMIZABLE TIP MAINTAINS THE DESIRED ANGLE
Allows the physician to straighten and reshape to the preferred angle and holds it throughout the procedure

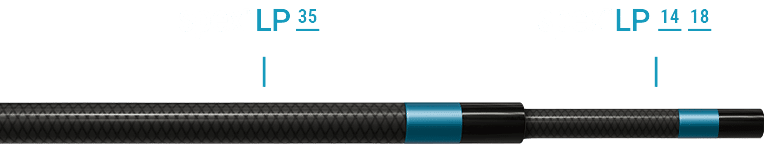
COMPATIBLE WITH YOUR PREFERRED GUIDEWIRE AND PROCEDURAL TECHNIQUE
Telescope SpexLP 18/ SpexLP 14 into the SpexLP 35 to maximize support.

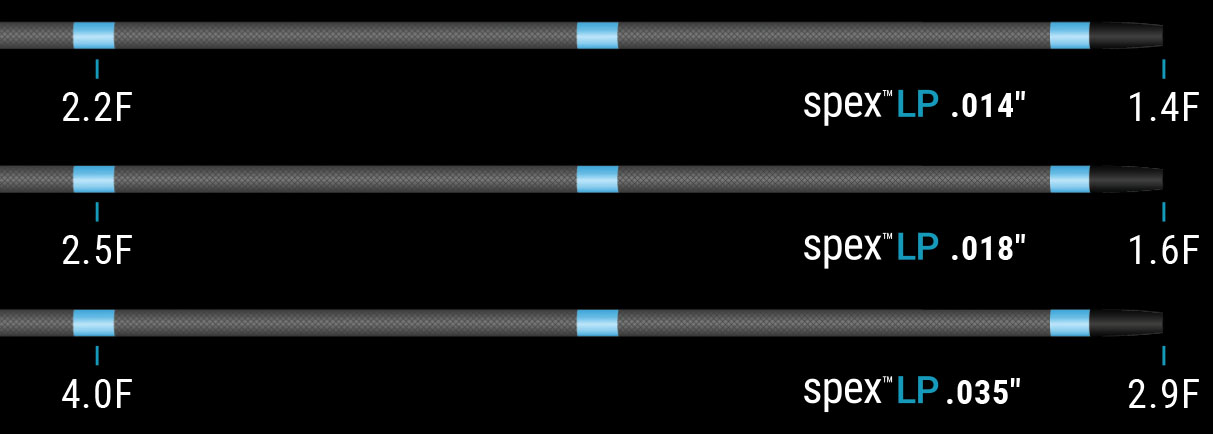


14
18
35
Model Number
SLP14090CE
SLP14090CE-5P*
SLP14135CE
SLP14135CE-5P*
SLP14150CE
SLP14150CE-5P*
SLP18090CE
SLP18090CE-5P*
SLP18135CE
SLP18135CE-5P*
SLP18150CE
SLP18150CE-5P*
SLP35090CE
SLP35090CE-5P*
SLP35135CE
SLP35135CE-5P*
SLP35150CE
SLP35150CE-5P*
Effective Length (cm)
90, 135,150
90, 135,150
90,135,150
Guidewire Compatability (in)
0.014″
0.018″
0.035”
Tip Profile
1.4F
1.6F
2.9F
Max Outer Diameter (F)
2.2F
2.5F
4.0F
Max Pressure (psi/kpa)
360/ 2482
360/ 2482
360/ 2482
Shapeable Tip Zone (in/mm)
3.94/ 100
3.94/ 100
3.94/ 100
Sheath Compatibility (F/mm)
4/ 1.3
4/ 1.3
5 / 1.7
Hydrophilic Coating (cm)
40
40
40
*(-5P) Indicates 5 pack availability
©2024 Reflow Medical, Inc. All rights reserved. Reflow Medical, Spex, and The Pulse of Medical Ingenuity are registered trademarks or trademarks of Reflow Medical, Inc. Federal law (USA) restricts these devices to sale by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the Indications, Contraindications, Warnings, Precautions, Complications, and Directions for Use.
Indications, Safety, & Warnings
CAUTION: This device is restricted to use by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the Indications, Contraindications, Warnings, Precautions, Adverse Events, and Procedural Steps.
Intended Use
The Spex Shapeable Support Microcatheter is intended to treat peripheral artery disease (PAD).
Indications for Use
The Spex™ Shapeable Support Microcatheter is intended to be used in conjunction with steerable guidewires to access discrete regions of the peripheral vasculature. To facilitate access in conjunction with a guidewire, it may be desired to shape the tip of the microcatheter. They may be used to facilitate placement and exchange of guidewires and other interventional devices and provide a conduit for delivery of saline solutions or diagnostic/ therapeutic agents.
Contraindications
The Spex Shapeable Support Microcatheter is contraindicated for use in the coronary and cerebral vasculature.
Warnings
• Single Use only. Do not reuse/resterilize. Reusing the device could result in compromised device performance, cross-infection and other safety related hazards including patient injury.
• Do not use if device is open or packaging is damaged.
• Never advance, withdraw or rotate an intravascular device against resistance until the cause is determined by fluoroscopy
• If the catheter is damaged, this product may cut into a blood vessel wall. Extreme caution needs to be taken when removing a damaged device. In the case of complications resulting from the removal of the entire system, stop immediately the procedure, and perform appropriate treatment at the discretion of the physician.
Precautions
• Store in a cool, dry, dark place. Storage of the device in extreme conditions may damage the device and/or affect device performance that could lead to patient injury.
• Use only appropriately sized ancillary device, as shown in the Specifications above.
• Maximum Injection Pressure: 360 psi (2482kpa).
• Use the catheter prior to the “Use By” date specified on the package.
• The catheter should only be used by physicians qualified to perform percutaneous vascular interventions.
• Precautions to prevent or reduce clotting should be taken when any catheter is used in the vascular system.
• Use of systemic heparinization and heparinized saline solution should be considered.
• Exercise care while handling the microcatheter during procedure to reduce the possibly of accidental damage, kinking or bending.
• Manipulation of the microcatheter should only occur under fluoroscopy.
©2024 Reflow Medical, Inc. All rights reserved. Reflow Medical, Spex and The Pulse of Medical Ingenuity are registered trademarks or trademarks of Reflow Medical, Inc. This device is restricted to use by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the Indications, Contraindications, Warnings, Precautions, Adverse Events, and Procedural Steps.
QUESTIONS?
TALK TO A REFLOW PRO.
For more information, please send us your question or request today.
A Reflow pro will get back to you.



